Introduction
Accurate oxygen measurement is critical in many industrial processes, affecting performance, safety, and environmental compliance. Zirconia oxygen sensors, known for their precision and durability, are widely used in applications like combustion systems and fuel cells to measure oxygen partial pressure. But how do these sensors work, and why is zirconia the preferred material? In this blog, we’ll provide a concise overview of the principles behind zirconia oxygen sensors and explore their applications across various industries, offering key technical insights.
Table of Content
1. How Does a Zirconia Oxygen Sensor Work?
2. Zirconia Oxygen Sensor Equation Explanation
3. Isweek Zirconia Oxygen Sensor
4. How to calculate oxygen partial pressure?
4.1 What is partial pressure?
4.2 Dalton’s Law: Physical Principle
1. How Does a Zirconia Oxygen Sensor Work?
The zirconia oxygen sensor is designed based on the oxygen ion conductivity characteristic of stabilized zirconia ceramic when exposed to an environment above 650°C. Under certain temperature conditions, if there are different oxygen partial pressures (i.e., oxygen concentrations) on either side of the zirconia ceramic block, a series of reactions and the migration of oxygen ions will occur within the zirconia ceramic. At this point, a stable millivolt-level signal can be measured through the electrodes on both sides of the zirconia, referred to as the oxygen potential.
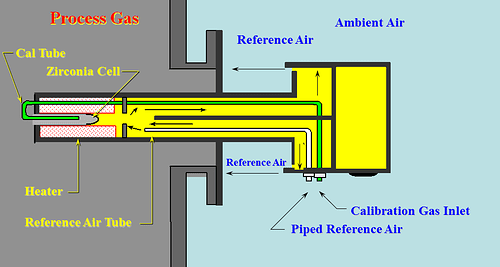 Figure1-Zirconia Oxygen Sensor working
Figure1-Zirconia Oxygen Sensor working
2. Zirconia Oxygen Sensor Equation Explanation
It adheres to the principles of the Nernst equation: E is the oxygen potential output by the oxygen sensor (in mV),_kTk is the absolute temperature inside the furnace (in K), and P1 and P2 are the oxygen partial pressures on either side of the zirconia. In practical applications, one side of the zirconia is exposed to a gas with a known oxygen concentration (usually air), which we refer to as the reference gas. The other side is exposed to the gas to be measured, which is the atmosphere inside the furnace that we want to detect. The signal output by the oxygen sensor is the oxygen potential signal. Using the Nernst equation, we can determine the relationship between the oxygen partial pressure in the furnace atmosphere and the oxygen potential. When the reference gas is air, it can be expressed as follows: E represents the oxygen potential output by the oxygen sensor, Tk is the absolute temperature inside the furnace, and PO2 is the oxygen partial pressure in the furnace.
3. Isweek Zirconia Oxygen Sensor
The SST oxygen sensors distributed by Isweek.com come with a self-heating device that typically maintains the temperature at around 700°C, ensuring that the value of Tk remains stable. This stability allows for the direct measurement of the oxygen partial pressure concentration within the furnace using the above equation. In engineering applications, standard gases are used to calibrate the relationship between the oxygen potential output E of the oxygen sensor and the oxygen partial pressure concentration PO2P. This calibration method is currently recognized as the most accurate and direct approach. The SST zirconia oxygen sensor system – O2S-FR-T2-18BM-C employs both of these principles. It can measure oxygen concentration without the need for a reference gas. The core component is designed as a sandwich structure, consisting of two identical zirconia plates and three platinum rings, creating a sealed small chamber. One zirconia plate has electrodes on opposite sides and functions as an electrochemical oxygen pump. The other zirconia plate forms a Nernst voltage on its sides. Once the oxygen sensor is activated, the oxygen in the small chamber is evacuated, and at this point, the pressure P2P is proportional to the current it delivers.

Figure2-Zirconia Oxygen Sensor
On the other plate, the voltage increases. Once the voltage reaches a set reference value, the pump current changes direction, causing oxygen to be drawn back into the small chamber. As the pressure P2P rises to the set reference value, the pump current changes direction again. This process continuously repeats, with the length of the pump current reversal cycle being proportional to the oxygen pressure. This principle allows for the detection of oxygen partial pressure.
By eliminating the need for a sealed reference gas, the sensor becomes more versatile and can be used for various oxygen pressure measurements. When paired with our oxygen transmitter board, OXY-LC, this oxygen sensor can output both oxygen partial pressure and oxygen concentration values.
4. How to calculate oxygen partial pressure?
The working principle of a zirconia oxygen sensor is to measure the partial pressure of oxygen in a mixed gas. So, what is partial pressure? What is the principle and how to calculate it? And how is the oxygen partial pressure converted into the oxygen concentration in terms of volume content?
4.1 What is partial pressure?
Partial pressure is defined as the pressure exerted by an individual gas component in a mixed gas. It corresponds to the total pressure that would be exerted if that single gas component alone occupied the entire volume.
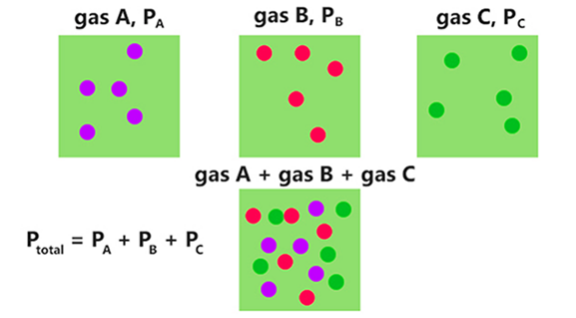
Figure3-partial pressure
4.2 Dalton’s Law: Physical Principle
The total pressure (Ptotal) of an ideal gas mixture is equal to the sum of the partial pressures (Pi) of the individual gases in the mixture.
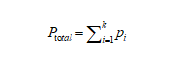
Figure4-formula1
From Equation 1, it can be derived that the ratio of the number of particles of a single gas component(ni)to the total number of particles in the gas mixture (ntotal) is equal to the ratio of the partial pressure of the single gas component(Pi) to the total pressure of the gas mixture (Ptotal)
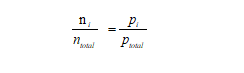
Figure5-formula2
ni: Number of particles of the gas component
ntotal : Total number of particles
pi : Partial pressure of the individual gas
Ptotal : Total pressure
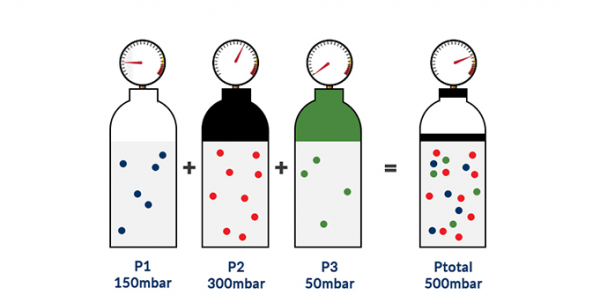
Figure6-temperature
Conversion of oxygen partial pressure to oxygen concentration in terms of volume content (using meteorological station records as an example).
- Temperature: 22°C
- Humidity: 32%
- Pressure: 986 mbar
Using the vapor pressure table above, WVPmax = 26.43mbar
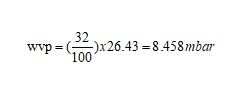
Figure7-formula3
Oxygen partial pressure:
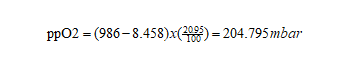
Figure8-formula3
By using the oxygen partial pressure and the total atmospheric pressure, we can calculate the volume content of oxygen.
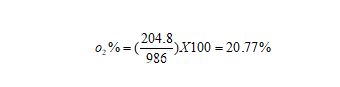
Figure9-formula4






