Introduction
A Zirconia Oxygen Sensor, often referred to simply as an oxygen sensor or O2 sensor, is a device utilized in automotive and industrial applications to measure the proportion of oxygen in exhaust gases or other environments. It is vital for ensuring the correct air-fuel ratio in internal combustion engines, which is essential for optimizing engine performance, fuel efficiency, and emissions control.
Table of Content
1. What is a Zirconia Oxygen Sensor?
2. How does the Zirconia Oxygen Sensor work?
3. Components ofZirconia Oxygen Sensor
4. How to prolong Zirconia Oxygen Sensorlife?
5. Applications of Zirconia Oxygen Sensor
6. Features of Zirconia Oxygen Sensor
7. FAQs of Zirconia Oxygen Sensor
1. What is a Zirconia Oxygen Sensor?
The SST Sensing zirconia oxygen sensors, distributed by Isweek, are utilized in combustion control systems, fuel combustion, and oxygen generation systems. The system comprises a fast-response zirconia (ZrO2) screw-in sensor and the OXY-LC interface board. The durable stainless steel sensor operates without needing a reference gas and functions effectively in extreme temperatures and harsh environments. It incorporates a unique closed-loop measurement system with a heartbeat signal for fault detection and can measure oxygen concentrations from 0.1% to 100%. The sensor is easy to install, operates at temperatures ranging from -100°C to 250°C, and has a lifespan of up to 10 years.

Figure1-zirconia oxygen sensors
2. How does the Zirconia Oxygen Sensor Work?
A zirconia oxygen sensor mainly comprises zirconia (ZrO2) and a protective sheath. There are two types of zirconia oxygen sensors: heated and non-heated.
The ceramic body of the zirconia tube is porous, allowing oxygen to permeate the solid electrolyte. At higher temperatures, the oxygen undergoes ionization. As long as there is a difference in oxygen concentration between the inside (atmosphere) and outside (exhaust) of the zirconia tube, oxygen ions will diffuse through the solid electrolyte from the atmosphere side to the exhaust side. This diffusion creates a micro-electrochemical cell, generating a voltage between the platinum electrodes on the zirconia tube.
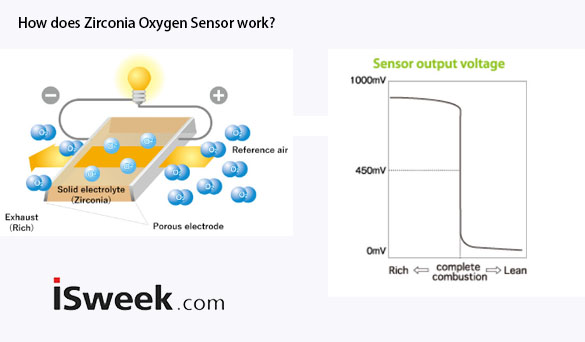
Figure2-Oxygen Sensor working principle
When the air-fuel mixture is lean, the exhaust contains more oxygen, leading to a reduced oxygen concentration difference across the zirconia tube and a lower voltage. In contrast, in a rich mixture, the exhaust has lower oxygen levels and higher concentrations of CO, CH, and NOx. These elements interact with the remaining oxygen on the zirconia tube’s outer surface, facilitated by the platinum catalyst, diminishing the oxygen concentration on the outer surface to zero. This abrupt elevation in the oxygen concentration difference across the zirconia tube substantially increases the voltage produced between the electrodes.
Therefore, the voltage generated by the oxygen sensor exhibits a sharp change around the air-fuel ratio coefficient λ = 1. When λ is greater than 1, the oxygen sensor output voltage is nearly zero, whereas when λ is less than 1, the output voltage approaches 1V. In the engine’s closed-loop control of the air-fuel mixture, the oxygen sensor works like a concentration switch. It sends varying-width electrical pulse signals to the ECU based on changes in the air-fuel ratio. The ECU uses the feedback signal from the oxygen sensor to adjust the fuel injection amount, thereby minimizing the harmful components in the exhaust gases.
3. Components of Zirconia Oxygen Sensor
The core of the zirconia oxygen sensor is the sensing unit (see Figure 3). This unit consists of two square-shaped zirconia (ZrO2) elements, each coated with a thin, porous layer of platinum. The platinum layer serves as the electrode, providing the necessary catalytic action for the dissociation of oxygen, allowing oxygen ions to move in and out of the zirconia.
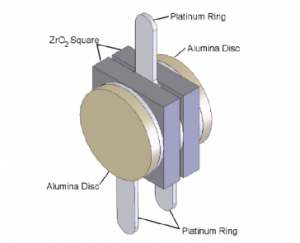
Figure3 sensing unit
The two square zirconia discs are positioned apart by a platinum ring, creating a sealed sensing chamber. Two extra platinum rings, in addition to the central platinum ring, are located on the outer surface of the zirconia to facilitate the electrical connections for the zirconia center (Pump Common Sense).
The two outer alumina (Al2O3) discs function as filters, effectively blocking environmental particulates and unburned gases from reaching the sensor. This protective measure is crucial in preventing contamination of the cell, which in turn ensures the stability of measurement readings. Figure 4 illustrates a cross-section of the sensing unit, providing a detailed view of its key components.
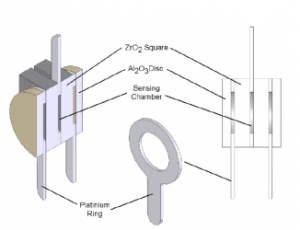
Figure4-outer alumina (Al2O3)
The zirconia core component is surrounded by a heating coil that generates the necessary operating temperature of 700°C. Subsequently, the zirconia core and heating coil are installed within a porous stainless steel protective cover. This cover filters out larger particles and dust, safeguarding the sensor from mechanical damage. Figure 5 illustrates the complete sensor assembly.
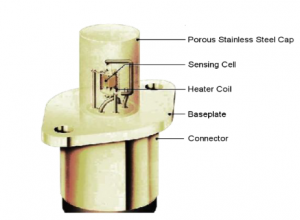
Figure5-heating coil
Pump Disc (First Zirconia Block)
The initial zirconia block serves as an electrochemical oxygen pump, utilized for either removing or reintroducing oxygen into the sensing chamber. The movement of oxygen ions through the zirconia block from one electrode to the other is dependent on the direction of the direct current (DC) power supply, consequently modifying the oxygen concentration and oxygen pressure (P2) within the enclosed chamber. The pumping procedure is regulated to maintain a partial oxygen pressure inside the sealed chamber that is consistently lower than the ambient oxygen pressure outside the chamber. The electrical connections of the zirconia core component are illustrated in Figure 6.
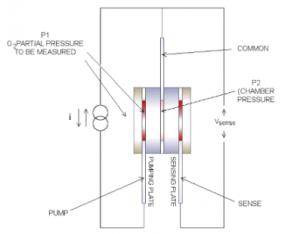
Figure 6- first zirconia block
Sensing Disc (Second Zirconia Block)
A Nernst voltage is generated due to the oxygen pressure difference across the second zirconia block, and this voltage is logarithmically proportional to the ratio of oxygen ion concentration. Given the oxygen pressure (P₁) within the sealed chamber, the induced voltage relative to the common line is always positive.
By measuring this voltage and comparing it with two reference voltages, the direction of the constant current source is reversed each time either of these reference voltages is reached. When the partial oxygen pressure is higher, it takes longer to reach the pump reversal voltage than it does in an atmosphere with lower oxygen partial pressure. This is because more oxygen ions need to be pumped to generate the same proportional pressure difference across the sensing disc.
Case:
The pressure of oxygen that requires measurement, denoted as P1, aligns with the set reference voltage at 10 mbar, while P2 registers at 5 mbar. If P1 is adjusted to 1 bar, P2 must be set at 0.5 bar to maintain the same reference voltage. This adjustment necessitates the removal of additional oxygen ions. Given that the current source responsible for ion pumping remains constant, the process will consequently take a longer duration to complete.
4. How to prolong Zirconia Oxygen Sensorlife?
A frequently asked question is how to extend the life of a zirconia oxygen sensor. While there are several measures we can take, it is crucial to understand and avoid certain chemicals and gases that may contaminate the sensor, leading to premature replacement.
Many oxygen sensors are subject to cross-sensitivity issues, so identifying the root cause of these sensitivities is essential for avoiding environments that could pollute the zirconium dioxide oxygen sensor.
Specific gases and chemicals can adversely affect our zirconium dioxide oxygen sensors, impacting their lifespan and performance. In this section, we will outline strategies to ensure the longevity of oxygen sensors and provide detailed information on which gases and chemicals to avoid to maintain optimal sensor performance.

Figure 7 Prolong Zirconia Oxygen Sensor
Combustible Gases
Small amounts of combustible gases may burn on the hot Pt-electrode surfaces or Al₂O₃ filters of the sensor. Generally, this combustion will be stoichiometric as long as sufficient oxygen is available. However, the sensor will measure the residual oxygen pressure, leading to potential measurement errors. Therefore, the sensor is not recommended for applications involving large amounts of combustible gases where precise oxygen measurement is critical. The gases investigated by Week are as follows:
- H₂(Hydrogen) up to 2%; stoichiometric combustion
- CO (Carbon Monoxide) up to 2%; stoichiometric combustion
- CH₄ (Methane) up to 2.5%; stoichiometric combustion
- NH₃ (Ammonia) up to 1500ppm; stoichiometric combustion
Heavy Metals
Vapors from metals such as zinc (Zn), cadmium (Cd), lead (Pb), and bismuth (Bi) can impact the catalytic properties of the Pt-electrodes. It is essential to avoid exposing Zirconium Dioxide Oxygen Sensors to these metal vapors.
Halogen and Sulphur Compounds
Small amounts (less than 100 ppm) of halogens and/or sulfur compounds do not affect the performance of the oxygen sensors. However, higher concentrations of these gases can eventually cause readout issues or, particularly in condensing environments, lead to corrosion of sensor components. Gases investigated by Week are listed below;
- Halogens, F₂ (Fluorine), Cl₂ (Chlorine)
- HCL (Hydrogen Chloride), HF (Hydrogen Fluoride)
- SO₂ (Sulphur Dioxide)
- H₂S (Hydrogen Sulphide)
- Freon gases
- CS₂ (Carbon Disulfide)
Reducing Atmospheres
Prolonged exposure to reducing atmospheres can gradually diminish the catalytic effectiveness of the Pt-electrodes and should be avoided. Reducing atmospheres are characterized by very low levels of free oxygen and the presence of combustible gases, where oxygen is consumed as these gases burn.
Many customers often confuse ISWeek’s oxygen sensors with Bosch Lambda sensors, which are primarily used in automotive combustion applications. However, Week’s oxygen sensors are specifically designed for boiler combustion control. Life tests have been conducted in the following environments:
- A laboratory atmosphere
- Exhaust gases of natural gas-fired boilers
- Exhaust gases of light oil
5. Applications of Zirconia Oxygen Sensor
Industries globally have acknowledged the significance of zirconia oxygen sensors. These sensors play a crucial role in various applications, ranging from combustion control in power plants to the maintenance of ideal conditions in kilns, thereby contributing to the smooth and efficient operation of these systems.
Automotive and Motorcycle
Automotive engines utilize zirconia oxygen sensors, typically positioned in the exhaust system, to gauge the oxygen levels in the exhaust fumes. This information is essential for the engine control unit (ECU) to regulate the air-fuel ratio and enhance engine efficiency.
Figure8-Automotive and Motorcycle
Industry Field
- Power Plants: Power plants, especially those burning fossil fuels, require close monitoring and control of the combustion process to maximize efficiency and minimize emissions. Here, zirconia oxygen sensors provide an accurate and durable solution.
- Food and Beverage Industry: In food packaging, zirconia oxygen sensors are used to monitor oxygen levels and ensure the shelf life and quality of the products.
6. Features of Zirconia Oxygen Sensor
- Zirconium dioxide technology
- Interface electronics included
- Mounting positions available; 28mm, 45mm, and 55mm
- 5 screw mounting
- All stainless steel construction, both internally and externally
- Long life
- No reference gas is required creating the ability to measure a wide oxygen range
- Non-depleting technology
- Linear output signal
- No need for temperature stabilization
- It can be used in high-pressure and temperature environments
- Simple recalibration if/when required
- Full technical and application support is available
7. FAQs of Zirconia Oxygen Sensor
What is the difference between zirconia and titanium O2 sensors?
Titanium sensors do not produce their voltage like Zirconia sensors. Instead, they operate by altering the resistance of the sensing element based on the amount of oxygen in the exhaust gases.
- What is the lifetime of the zirconia sensor?
The Probe zirconia oxygen sensor provides a linear output signal, has a lifespan of up to 10 years (depending on the specific application), and measures oxygen levels ranging from 0.1% to 100% O₂.
- Why zirconia is better?
A major benefit of zirconia is its robustness and longevity. Think about the amount of pressure your molars apply when you chew food. Your crowns need to be constructed from a sturdy material, making zirconia a suitable option for crowns located at the back of your mouth.
- How many wires does a zirconia oxygen sensor have?
Zirconia oxygen sensors come in various models that can be distinguished by their wire count: a 1-wire model is a non-heated sensor that uses the exhaust system for its ground signal; a 3-wire model is a heated sensor that also grounds through the exhaust system; and a 4-wire model is a heated sensor with a separate ground wire, typically linked to the ECU.






